
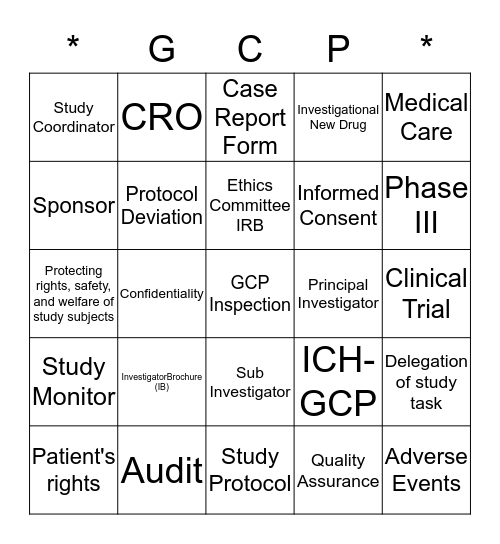
This bingo card has 25 words: InvestigatorBrochure (IB), Study Protocol, Ethics Committee IRB, Patient's rights, Medical Care, Study Coordinator, Sponsor, CRO, Principal Investigator, Sub Investigator, Informed Consent, Protecting rights, safety, and welfare of study subjects, ICH-GCP, Delegation of study task, Protocol Deviation, Adverse Events, Clinical Trial, Case Report Form, Phase III, Confidentiality, GCP Inspection, Audit, Study Monitor, Investigational New Drug and Quality Assurance.
Clinical Research BINGO | ICH-GCP | Investigator Responsibilities | Clinical Research BINGO | GCP Bingo
Share this URL with your players:
For more control of your online game, create a clone of this card first.
Learn how to conduct a bingo game.
With players vying for a you'll have to call about __ items before someone wins. There's a __% chance that a lucky player would win after calling __ items.
Tip: If you want your game to last longer (on average), add more unique words/images to it.