
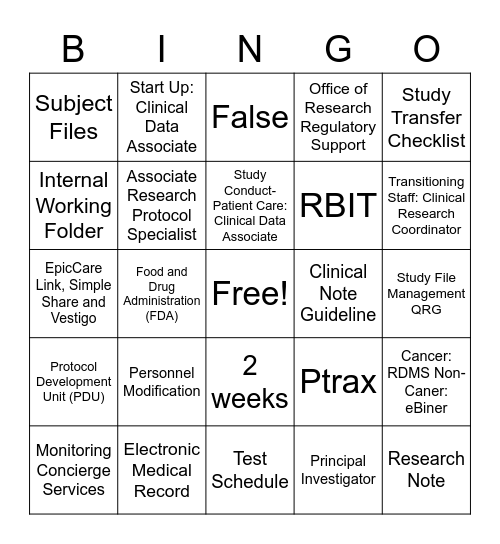
This bingo card has a free space and 32 words: Principal Investigator, Contract Research Organization, Food and Drug Administration (FDA), Institutional Review Board, Protocol Development Unit (PDU), Associate Research Protocol Specialist, Study Transfer Checklist, Cancer: RDMS Non-Caner: eBiner, Start Up: Clinical Data Associate, Source Documents, Study Conduct-Patient Care: Clinical Data Associate, False, Study Conduct: Clinical Research Coordinator, Study Conduct-Patient Care: Clinical Data Associate, Clinical Note Guideline, Study Conduct-Oversight, Maintenance, and Reporting: Clinical Data Associate, Monitoring Concierge Services, EpicCare Link, Simple Share and Vestigo, 2 weeks, Personnel Modification, Ptrax, 48 hours, Study File Management QRG, Electronic Medical Record, Research Note, Office of Research Regulatory Support, RBIT, Internal Working Folder, Subject Files, Test Schedule, Supervisor and Clinical Research Coordinator and Transitioning Staff: Clinical Research Coordinator.
⚠ This card has duplicate items: Study Conduct-Patient Care: Clinical Data Associate (2)
Clinical Trials BINGO | Health Information and Related Careers | Health Information and Related Careers | PHACS Bingo | Research Brainstorm BINGO!
Share this URL with your players:
For more control of your online game, create a clone of this card first.
Learn how to conduct a bingo game.
With players vying for a you'll have to call about __ items before someone wins. There's a __% chance that a lucky player would win after calling __ items.
Tip: If you want your game to last longer (on average), add more unique words/images to it.