
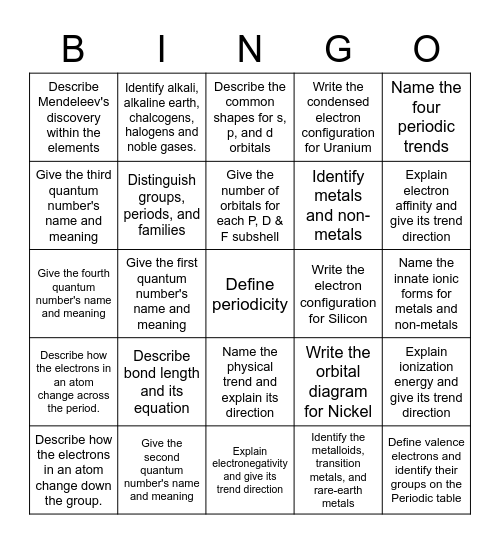
This bingo card has a free space and 24 words: Describe bond length and its equation, Give the second quantum number's name and meaning, Give the number of orbitals for each P, D & F subshell, Describe how the electrons in an atom change down the group., Name the four periodic trends, Describe Mendeleev's discovery within the elements, Give the third quantum number's name and meaning, Identify alkali, alkaline earth, chalcogens, halogens and noble gases., Write the electron configuration for Silicon, Name the physical trend and explain its direction, Identify metals and non-metals, Give the fourth quantum number's name and meaning, Write the orbital diagram for Nickel, Explain ionization energy and give its trend direction, Distinguish groups, periods, and families, Identify the metalloids, transition metals, and rare-earth metals, Name the innate ionic forms for metals and non-metals, Describe the common shapes for s, p, and d orbitals, Explain electron affinity and give its trend direction, Give the first quantum number's name and meaning, Define valence electrons and identify their groups on the Periodic table, Describe how the electrons in an atom change across the period., Write the condensed electron configuration for Uranium and Explain electronegativity and give its trend direction.
Unit 2 - Atomic Structure | Atom & Periodic Table | Atom & Periodic Table | Atom & Periodic Table | Atom & Periodic Table
Share this URL with your players:
For more control of your online game, create a clone of this card first.
Learn how to conduct a bingo game.
With players vying for a you'll have to call about __ items before someone wins. There's a __% chance that a lucky player would win after calling __ items.
Tip: If you want your game to last longer (on average), add more unique words/images to it.