
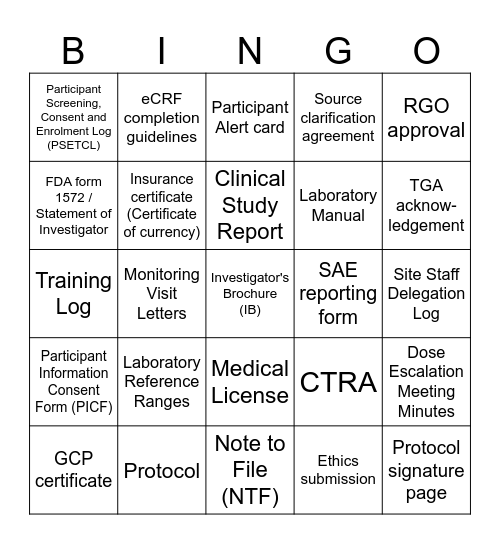
This bingo card has 25 words: Participant Information Consent Form (PICF), Protocol, Investigator's Brochure (IB), Ethics submission, RGO approval, Participant Screening, Consent and Enrolment Log (PSETCL), eCRF completion guidelines, Participant Alert card, SAE reporting form, Dose Escalation Meeting Minutes, FDA form 1572 / Statement of Investigator, Insurance certificate (Certificate of currency), Note to File (NTF), CTRA, TGA acknow-ledgement, GCP certificate, Monitoring Visit Letters, Clinical Study Report, Laboratory Manual, Site Staff Delegation Log, Training Log, Laboratory Reference Ranges, Medical License, Source clarification agreement and Protocol signature page.
IRB Bingo | Clinical Research | Qualitative Bingo | Unit 3 Terms 2 | Unit 3 Terms 2
Share this URL with your players:
For more control of your online game, create a clone of this card first.
Learn how to conduct a bingo game.
With players vying for a you'll have to call about __ items before someone wins. There's a __% chance that a lucky player would win after calling __ items.
Tip: If you want your game to last longer (on average), add more unique words/images to it.