Coulomb's Law Use of Mass Spectroscopy to discover isotopes Schrodinger Democritus Formulated the "Atomic Theory of Matter" Newlands Planck Thomson Mendeleev the "Law of Conservation of Matter" Seaborg Uncertainty Principle Rutherford's Atomic Model Planetary Model of the Atom Oil Drop Experiment Joseph Proust Wave Particle Duality Chadwick Photoelectric Effect Law of Definite Proportions Moseley Coulomb's Law Use of Mass Spectroscopy to discover isotopes Schrodinger Democritus Formulated the "Atomic Theory of Matter" Newlands Planck Thomson Mendeleev the "Law of Conservation of Matter" Seaborg Uncertainty Principle Rutherford's Atomic Model Planetary Model of the Atom Oil Drop Experiment Joseph Proust Wave Particle Duality Chadwick Photoelectric Effect Law of Definite Proportions Moseley
(Print)
Coulomb's Law
Use of Mass Spectroscopy to discover isotopes
Schrodinger
Democritus
Formulated the "Atomic Theory of Matter"
Newlands
Planck
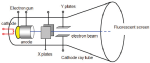
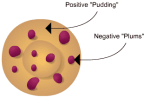
Thomson
Mendeleev
the "Law of Conservation of Matter"
Seaborg
Uncertainty Principle
Rutherford's Atomic Model
Planetary Model of the Atom
Oil Drop Experiment
Joseph Proust
Wave Particle Duality
Chadwick
Photoelectric Effect
Law of Definite Proportions
Moseley